GxP Templates |
Cleanroom Tools
Zener Engineering Services Ltd
GxP Templates |
Cleanroom Tools
Zener Engineering Services Ltd
GxP Templates Cleanroom Tools
Zener Engineering Services Ltd
Click Below For ZES Products
Click Below For ZES Products
GxP Policies, SOPs And Validation Templates For Sale
Zener Engineering Services Ltd (ZES)
Life Science document templates are available for purchase. Most of our templates contain help and guidance text in blue and are in MS Word compatible format. Delivery shall be via email and can take up to 14 days following receipt of payment. Terms and Conditions apply to the ZES products for sale.
All templates are in English and stated document page numbers are approximate. Scroll down the page for the following:
- Quality Management System
- Validation Templates (3 sets)
- Training Document Templates
- Engineering Standards Templates
- Various Policies and Standard Operating Procedures (SOPs) Templates
Validation Template Set purchase includes 1 hour of free technical support supplied via email, phone or Zoom.
GxP Policies, SOPs And Validation Templates For Sale
Zener Engineering Services Ltd (ZES)
Life Science document templates are available for purchase. Most of our templates contain help and guidance text in blue and are in MS Word compatible format. Delivery shall be via email and can take up to 14 days following receipt of payment. Terms and Conditions apply to the ZES products for sale.
All templates are in English and stated document page numbers are approximate. Scroll down the page for the following:
- Quality Management System
- Validation Templates (3 sets)
- Training Document Templates
- Engineering Standards Templates
- Various Policies and Standard Operating Procedures (SOPs) Templates
Validation Template Set purchase includes 1 hour
of free technical support
supplied via email, phone or Zoom.
GxP Policies, SOPs And
Validation Templates
For Sale
Zener Engineering Services (ZES)
Life Science document templates are available for purchase. Most of our templates contain help and guidance text in blue and are in MS Word compatible format. Delivery shall be via email and can take up to 14 days following receipt of payment. Terms and Conditions apply to the ZES products for sale.
All templates are in English and stated document page numbers are approximate. Scroll down the page for the following:
- Quality Management System
- Validation Templates (3 sets)
- Training Document Templates
- Engineering Standards Templates
- Various Policies and Standard Operating Procedures (SOPs) Templates
Validation Template Set purchase
includes 1 hour
of free technical support
supplied via email, phone or Zoom.
Quality Management System Templates (Various)
Template CatalogueThe Quality Manual is the first level of documentation in any Life Science company's Quality Management System.
The ZES Quality Manual draft describes the scope of a QMS and provides documented evidence of an organisation’s quality policies, which shows that the organisation is committed to a quality culture.
Templates Available:
- Quality Manual
- Change Control
- Standard Operating Procedure
Download the Template Catalogue below for more details
Computer System Validation Templates Set 1
Template CatalogueThe set of comprehensive computer system validation templates. Guidance text in Blue to aid completion.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details
Computer System Validation Templates Set 2
Template CatalogueStreamlined set of CSV templates for the smaller or start-up company.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details
Equipment Validation Templates Set 3
Template CatalogueEquipment Validation Templates.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details
GxP Policies And SOPs (Various)
Template CatalogueVarious Including:
- CSV Risk Management
- CSV Software Validation
- Medical Device Cyber Security Functionality Statement Form
- Computer Systems Validation Gap Analysis
- Configuration Management Policy
and many more...
Download the Template Catalogue below for more details
GMP Engineering Standards (Various)
Template CatalogueVarious Including:
- Coding Standard
- Electrical Panel etc
- WFI Water Systems etc
and many more...
Download the Template Catalogue below for more details
Download the Template Catalogue below for more details and prices.
Can't find what you are looking for?
ZES have numerous policies, procedures and templates, many not listed here.
Contact ZES with your particular requirements and we will be sure to help.
Quality Management System Templates (Various)
Catalogue DownloadThe Quality Manual is the first level of documentation in any Life Science company's Quality Management System. The ZES Quality Manual draft describes the scope of a QMS and provides documented evidence of an organisation’s quality policies, which shows that the organization is committed to a quality culture.
Templates Available:
1. Quality Manual
2. Change Control
3. Standard Operating Procedure
Download the Template Catalogue below for more details.
Computer System Validation Templates Set 1
Catalogue DownloadA set of comprehensive computer system validation templates. Guidance text in Blue to aid completion.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details.
Computer System Validation Templates Set 2
Catalogue DownloadStreamlined set of CSV templates for the smaller or start-up company.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details.
Equipment Validation Templates Set 3
Catalogue DownloadEquipment Validation Templates.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details.
GxP Policies And SOPs (Various)
Catalogue DownloadVarious Templates Available Including:
1. CSV Risk Management
2. CSV Software Validation
3. Medical Device Cyber Security
4. Functionality Statement Form
5. Computer Systems Validation Gap Analysis
6. Configuration Management Policy
and many more...
Download the Template Catalogue below for more details.
GMP Engineering Standards (Various)
Catalogue DownloadVarious Templates Available Including:
1. Coding Standard
2. Electrical Panel etc
3. WFI Water Systems etc
and many more...
Download the Template Catalogue below for more details.
Download the Template Catalogue below for more details and prices.
Can't find what you are looking for?
ZES have numerous policies, procedures and templates, many not listed here.
Contact ZES with your particular requirements and we will be sure to help.
Quality Management System Templates (Various)
Catalogue DownloadThe Quality Manual is the first level of documentation in any Life Science company's Quality Management System. The ZES Quality Manual draft describes the scope of a QMS and provides documented evidence of an organisation’s quality policies, which shows that the organization is committed to a quality culture.
Templates Available:
1. Quality Manual
2. Change Control
3. Standard Operating Procedure
Download the Template Catalogue below for more details.
Computer System Validation Templates Set 1
Catalogue DownloadA set of comprehensive computer system validation templates. Guidance text in Blue to aid completion.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details.
Computer System Validation Templates Set 2
Catalogue DownloadStreamlined set of CSV templates for the smaller or start-up company.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details.
Equipment Validation Templates Set 3
Catalogue DownloadEquipment Validation Templates.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details.
GxP Policies And SOPs (Various)
Catalogue DownloadVarious Templates Available Including:
1. CSV Risk Management
2. CSV Software Validation
3. Medical Device Cyber Security 4. Functionality Statement Form
5. Computer Systems Validation Gap Analysis
6. Configuration Management Policy
Download the Template Catalogue below for more details.
GMP Engineering Standards (Various)
Catalogue DownloadVarious Templates Available Including:
1. Coding Standard
2. Electrical Panel etc
3. WFI Water Systems etc
Download the Template Catalogue below for more details.
Quality Management System Templates (Various)
Catalogue DownloadThe Quality Manual is the first level of documentation in any Life Science company's Quality Management System. The ZES Quality Manual draft describes the scope of a QMS and provides documented evidence of an organisation’s quality policies, which shows that the organization is committed to a quality culture.
Templates Available:
1. Quality Manual
2. Change Control
3. Standard Operating Procedure
Download the Template Catalogue below for more details.
Computer System Validation Templates Set 1
Catalogue DownloadA set of comprehensive computer system validation templates. Guidance text in Blue to aid completion.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details.
Computer System Validation Templates Set 2
Catalogue DownloadStreamlined set of CSV templates for the smaller or start-up company.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details.
Equipment Validation Templates Set 3
Catalogue DownloadEquipment Validation Templates.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details.
GxP Policies And SOPs (Various)
Catalogue DownloadVarious Templates Available Including:
1. CSV Risk Management
2. CSV Software Validation
3. Medical Device Cyber Security 4. Functionality Statement Form
5. Computer Systems Validation Gap Analysis
6. Configuration Management Policy
Download the Template Catalogue below for more details.
GMP Engineering Standards (Various)
Catalogue DownloadVarious Templates Available Including:
1. Coding Standard
2. Electrical Panel etc
3. WFI Water Systems etc
Download the Template Catalogue below for more details.
Quality Management System Templates (Various)
Catalogue DownloadThe Quality Manual is the first level of documentation in any Life Science company's Quality Management System. The ZES Quality Manual draft describes the scope of a QMS and provides documented evidence of an organisation’s quality policies, which shows that the organization is committed to a quality culture.
Templates Available:
1. Quality Manual
2. Change Control
3. Standard Operating Procedure
Download the Template Catalogue below for more details.
Computer System Validation Templates Set 1
Catalogue DownloadA set of comprehensive computer system validation templates. Guidance text in Blue to aid completion.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details.
Computer System Validation Templates Set 2
Catalogue DownloadStreamlined set of CSV templates for the smaller or start-up company.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details.
Equipment Validation Templates Set 3
Catalogue DownloadEquipment Validation Templates.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details.
GxP Policies And SOPs (Various)
Catalogue DownloadVarious Templates Available Including:
1. CSV Risk Management
2. CSV Software Validation
3. Medical Device Cyber Security 4. Functionality Statement Form
5. Computer Systems Validation Gap Analysis
6. Configuration Management Policy
Download the Template Catalogue below for more details.
GMP Engineering Standards (Various)
Catalogue DownloadVarious Templates Available Including:
1. Coding Standard
2. Electrical Panel etc
3. WFI Water Systems etc
Download the Template Catalogue below for more details.
Quality Management System Templates (Various)
Catalogue DownloadThe Quality Manual is the first level of documentation in any Life Science company's Quality Management System. The ZES Quality Manual draft describes the scope of a QMS and provides documented evidence of an organisation’s quality policies, which shows that the organization is committed to a quality culture.
Templates Available:
1. Quality Manual
2. Change Control
3. Standard Operating Procedure
Download the Template Catalogue below for more details.
Computer System Validation Templates Set 1
Catalogue DownloadA set of comprehensive computer system validation templates. Guidance text in Blue to aid completion.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details.
Computer System Validation Templates Set 2
Catalogue DownloadStreamlined set of CSV templates for the smaller or start-up company.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details.
Equipment Validation Templates Set 3
Catalogue DownloadEquipment Validation Templates.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details.
GxP Policies And SOPs (Various)
Catalogue DownloadVarious Templates Available Including:
1. CSV Risk Management
2. CSV Software Validation
3. Medical Device Cyber Security 4. Functionality Statement Form
5. Computer Systems Validation Gap Analysis
6. Configuration Management Policy
Download the Template Catalogue below for more details.
GMP Engineering Standards (Various)
Catalogue DownloadVarious Templates Available Including:
1. Coding Standard
2. Electrical Panel etc
3. WFI Water Systems etc
Download the Template Catalogue below for more details.
Quality Management System Templates (Various)
Catalogue DownloadThe Quality Manual is the first level of documentation in any Life Science company's Quality Management System. The ZES Quality Manual draft describes the scope of a QMS and provides documented evidence of an organisation’s quality policies, which shows that the organization is committed to a quality culture.
Templates Available:
1. Quality Manual
2. Change Control
3. Standard Operating Procedure
Download the Template Catalogue below for more details.
Computer System Validation Templates Set 1
Catalogue DownloadA set of comprehensive computer system validation templates. Guidance text in Blue to aid completion.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details.
Computer System Validation Templates Set 2
Catalogue DownloadStreamlined set of CSV templates for the smaller or start-up company.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details.
Equipment Validation Templates Set 3
Catalogue DownloadEquipment Validation Templates.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details.
GxP Policies And SOPs (Various)
Catalogue DownloadVarious Templates Available Including:
1. CSV Risk Management
2. CSV Software Validation
3. Medical Device Cyber Security
4. Functionality Statement Form
5. Computer Systems Validation Gap Analysis
6. Configuration Management Policy
Download the Template Catalogue below for more details.
GMP Engineering Standards (Various)
Catalogue DownloadVarious Templates Available Including:
1. Coding Standard
2. Electrical Panel etc
3. WFI Water Systems etc
Download the Template Catalogue below for more details.
Download the Template Catalogue below for more details and prices.
Can't find what you are looking for?
ZES have numerous policies, procedures and templates, many not listed here.
Contact ZES with your particular requirements and we will be sure to help.
Quality Management System Templates (Various)
Catalogue DownloadThe Quality Manual is the first level of documentation in any Life Science company's Quality Management System. The ZES Quality Manual draft describes the scope of a QMS and provides documented evidence of an organisation’s quality policies, which shows that the organization is committed to a quality culture.
Templates Available:
1. Quality Manual
2. Change Control
3. Standard Operating Procedure
Download the Template Catalogue below for more details.
Computer System Validation Templates Set 1
Catalogue DownloadA set of comprehensive computer system validation templates. Guidance text in Blue to aid completion.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details.
Computer System Validation Templates Set 2
Catalogue DownloadStreamlined set of CSV templates for the smaller or start-up company.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details.
Equipment Validation Templates Set 3
Catalogue DownloadEquipment Validation Templates.
The full life cycle is available from Plans to Reports.
Download the Template Catalogue below for more details.
GxP Policies And SOPs (Various)
Catalogue DownloadVarious Templates Available Including:
1. CSV Risk Management
2. CSV Software Validation
3. Medical Device Cyber Security 4. Functionality Statement Form
5. Computer Systems Validation Gap Analysis
6. Configuration Management Policy
Download the Template Catalogue below for more details.
GMP Engineering Standards (Various)
Catalogue DownloadVarious Templates Available Including:
1. Coding Standard
2. Electrical Panel etc
3. WFI Water Systems etc
Download the Template Catalogue below for more details.
Validation Template Sets For Sale
Cleanroom Stainless Steel Tools
Orders Now Being Taken
With no pores or cracks to harbour dirt, grime or bacteria, Stainless Steel lets a cleaning solution do all but the toughest cleaning jobs. Bacterial removal and ease of cleanability of all ZES Stainless Steel Cleanroom Tools helps protect the Cleanroom Environment.
ZES Cleanroom Tools Product Details:
• Precision Engineering
• AISI 420 Stainless Steel
• High Strength and Hardness
• Excellent Corrosion Resistance
• Excellent Value
• Free UK Delivery
ZES have a full range of Cleanroom Tools, manufactured to order.
Contact ZES For Your Cleanroom Tools Today!
Approved By GxP Engineers For GxP Engineers
Cleanroom Stainless Steel Tools
Orders Now Being Taken
With no pores or cracks to harbour dirt, grime or bacteria, Stainless Steel lets a cleaning solution do all but the toughest cleaning jobs. Bacterial removal and ease of cleanability of all ZES Stainless Steel Cleanroom Tools helps protect the Cleanroom Environment.
ZES Cleanroom Tools Product Details:
- Precision Engineering
- AISI 420 Stainless Steel
- High Strength and Hardness
- Excellent Corrosion Resistance
- Excellent Value
- Free UK Delivery
ZES have a full range of Cleanroom Tools, manufactured to order.
Contact ZES For Your Cleanroom Tools Today!
Cleanroom Stainless Steel Tools
Orders Now Being Taken
With no pores or cracks to harbour dirt, grime or bacteria, Stainless Steel lets a cleaning solution do all but the toughest cleaning jobs. Bacterial removal and ease of cleanability of all ZES Stainless Steel Cleanroom Tools helps protect the Cleanroom Environment.
ZES Cleanroom Tools Product Details:
ZES have a full range of Cleanroom Tools, manufactured to order.
Contact ZES For Your Cleanroom Tools Today!
Approved By GxP Engineers For GxP Engineers
Product Purchase Form
Thank you for registering for a ZES product
Please pay using the PayPAL icon below the product you have selected.
Please try again later.

Stainless Adjustable Wrench (Spanner)
AISI 420 Stainless Steel
High Strength and Hardness
Excellent Corrosion Resistance
Free UK Delivery
150mm In Stock
Various Sizes Available
Size Details
L: 150mm K:18mm
In Stock
Sizes Available
- L: 200mm K: 24mm
- L: 250mm K: 30mm
- L: 300mm K: 36mm
- L: 375mm K: 46mm
Use the Product Purchase Form to enquire on Pricing and Delivery for the sizes above
Quality Cleanroom Tools Supplied For Engineers By Engineers
£95.00 plus VAT @20%*
Terms and Conditions Apply

Stainless Side Cutters
AISI 420 Stainless Steel
High Strength and Hardness
Excellent Corrosion Resistance
Free UK Delivery
One Size Only 190mm
In Stock
Size L:190mm
Use the Product Purchase Form to enquire on Pricing and Delivery for the sizes above
Quality Cleanroom Tools Supplied For Engineers By Engineers
£175.00 plus VAT @20%*
Terms and Conditions Apply
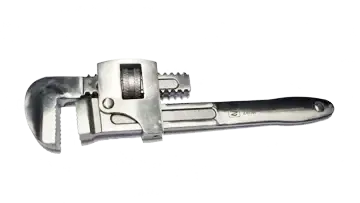
Stainless Pipe Wrench (Stilson)
AISI 420 Stainless Steel
High Strength and Hardness
Excellent Corrosion Resistance
Free UK Delivery
250mm In Stock
Various Sizes Available
Size Details
L: 250mm K: 35mm
In Stock
Sizes Available
L: 200mm
L: 250mm
L: 300mm
L: 375mm
Use the Product Purchase Form to enquire on Pricing and Delivery for the sizes above
Quality Cleanroom Tools Supplied For Engineers By Engineers
£175.00 plus VAT @20%*
Terms and Conditions Apply

Stainless Ratchet Wrench
AISI 420 Stainless Steel
High Strength and Hardness
Excellent Corrosion Resistance
Free UK Delivery
200mm 3/8 In Stock
Various Sizes Available
Sizes Available
L: 200 mm H:30 mm C: 3/8 in
L: 245 mm H:43 mm C: 1/2 in
L: 320 mm H:57 mm C: 3/4 in
L: 550 mm H:67 mm C:1/1 in
Use the Product Purchase Form to enquire on Pricing and Delivery for the sizes above
Quality Cleanroom Tools Supplied For Engineers By Engineers
£125.00 plus VAT @20%*
Terms and Conditions Apply

Stainless Combination Pliers
AISI 420 Stainless Steel
High Strength and Hardness
Excellent Corrosion Resistance
Free UK Delivery
150mm In Stock
Various Sizes Available
Sizes Available
- 150mm
- 180mm
- 200mm
Use the Product Purchase Form to enquire on Pricing and Delivery for the sizes above
Quality Cleanroom Tools Supplied For Engineers By Engineers
£150.00 plus VAT @20%*
Terms and Conditions Apply
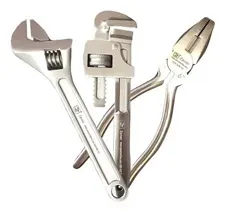
Contact ZES for the Full Product Range
AISI 420 Stainless Steel
High Strength and Hardness
Excellent Corrosion Resistance
Excellent Value
Free UK Delivery
Various Sizes Available

Stainless Adjustable Wrench (Spanner)
AISI 420 Stainless Steel
High Strength and Hardness
Excellent Corrosion Resistance
Free UK Delivery
150mm In Stock
Various Sizes Available
Size Details
L: 150mm K:18mm
In Stock
Sizes Available To Order
- L: 200mm K: 24mm
- L: 250mm K: 30mm
- L: 300mm K: 36mm
- L: 375mm K: 46mm
Use the Product Purchase Form to enquire on Pricing and Delivery for the sizes above
Quality Cleanroom Tools Supplied For Engineers By Engineers
£95.00 plus VAT @20%*
Terms and Conditions Apply

Stainless Side Cutters
AISI 420 Stainless Steel
High Strength and Hardness
Excellent Corrosion Resistance
Free UK Delivery
One Size Only 190mm
In Stock
Size L:190mm
Use the Product Purchase Form to enquire on Pricing and Delivery for the sizes above
Quality Cleanroom Tools Supplied For Engineers By Engineers
£175.00 plus VAT @20%*
Terms and Conditions Apply

Stainless Pipe Wrench (Stilson)
AISI 420 Stainless Steel
High Strength and Hardness
Excellent Corrosion Resistance
Free UK Delivery
250mm In Stock
Various Sizes Available
Size Details
L: 250mm K: 35mm
In Stock
Sizes Available To Order
L: 200mm
L: 250mm
L: 300mm
L: 375mm
Use the Product Purchase Form to enquire on Pricing and Delivery for the sizes above
Quality Cleanroom Tools Supplied For Engineers By Engineers
£175.00 plus VAT @20%*
Terms and Conditions Apply

Stainless Ratchet Wrench
AISI 420 Stainless Steel
High Strength and Hardness
Excellent Corrosion Resistance
Free UK Delivery
200mm 3/8 In Stock
Various Sizes Available
Wrench Sizes
L: 200mm H:30mm C:3/8in
L: 245mm H:43mm C:1/2in
L: 320mm H:57mm C:3/4in
L: 550mm H:67mm C:1/1in
Use the Product Purchase Form to enquire on Pricing and Delivery for the sizes above
Quality Cleanroom Tools Supplied For Engineers By Engineers
£125.00 plus VAT @20%*
Terms and Conditions Apply

Stainless Combination Pliers
AISI 420 Stainless Steel
High Strength and Hardness
Excellent Corrosion Resistance
Free UK Delivery
150mm In Stock
Various Sizes Available
Sizes Available
- 150mm
- 180mm
- 200mm
Use the Product Purchase Form to enquire on Pricing and Delivery for the sizes above
Quality Cleanroom Tools Supplied For Engineers By Engineers
£150.00 plus VAT @20%*
Terms and Conditions Apply

Contact ZES for the Full Product Range
AISI 420 Stainless Steel
High Strength and Hardness
Excellent Corrosion Resistance
Excellent Value
Free UK Delivery
Various Sizes Available
Product Purchase Form
Thank you for registering for a ZES product
Please pay using the PayPAL icon below the product you have selected.
Please try again later.
Coming Soon: Cleanroom Supplies
Coming Soon: Cleanroom Supplies
Qualification Frequently Asked Questions
Zener Engineering Services Ltd answer your GMP Qualification questions.
-
What is DQ IQ OQ PQ?
DQ, IQ, OQ, PQ protocols are methods for demonstrating that equipment being used or installed will offer a high degree of quality assurance such that production processes will consistently manufacture products that meet quality requirements.
- DQ stands for Design Qualification
- IQ stands for Installation Qualification
- OQ stands for Operational Qualification
- PQ stands for Performance Qualification
-
What is Design Qualification (DQ)?
Design Qualification (DQ) is a verification process that ensures the system design meets user requirements relating to system construction, output, quality and operation.
-
What is Installation Qualification (IQ)?
Installation Qualification (IQ) verifies that an instrument or unit of equipment being qualified (as well as its sub-systems and any ancillary systems) have been installed and configured according to the manufacturer’s specifications or installation checklist.
-
What is Operational Qualification (OQ)?
OQ’s purpose is to determine that equipment performance is consistent with the user requirement specification within the manufacturer-specified operating ranges.
System components are tested individually.
-
What is Performance Qualification (PQ)?
PQ's purpose is to ensure ongoing product quality by documenting performance over a period of time for certain processes.
PQ also provides evidence that all the individual system components, tested under OQ, work together.
Qualification Frequently Asked Questions
Zener Engineering Services Ltd answer your GMP Qualification questions.
-
What is DQ IQ OQ PQ?
DQ, IQ, OQ, PQ protocols are methods for demonstrating that equipment being used or installed will offer a high degree of quality assurance such that production processes will consistently manufacture products that meet quality requirements.
- DQ stands for Design Qualification
- IQ stands for Installation Qualification
- OQ stands for Operational Qualification
- PQ stands for Performance Qualification
-
What is Design Qualification (DQ)?
Design Qualification (DQ) is a verification process that ensures the system design meets user requirements relating to system construction, output, quality and operation.
-
What is Installation Qualification (IQ)?
Installation Qualification (IQ) verifies that an instrument or unit of equipment being qualified (as well as its sub-systems and any ancillary systems) have been installed and configured according to the manufacturer’s specifications or installation checklist.
-
What is Operational Qualification (OQ)?
OQ’s purpose is to determine that equipment performance is consistent with the user requirement specification within the manufacturer-specified operating ranges.
System components are tested individually.
-
What is Performance Qualification (PQ)?
PQ's purpose is to ensure ongoing product quality by documenting performance over a period of time for certain processes.
PQ also provides evidence that all the individual system components, tested under OQ, work together.
Can't find what you are looking for?
ZES have numerous policies, procedures and templates, many not listed here.
Contact ZES with your particular requirements and we will be sure to help.
Can't find what you are looking for?
ZES have numerous policies, procedures and templates,
many not listed here.
Contact ZES with your particular requirements
and we will be sure to help.
Can't find what you are looking for?
ZES have numerous policies, procedures and templates,
many not listed here.
Contact ZES with your particular requirements
and we will be sure to help.













